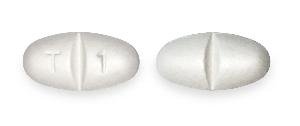
T 1 (Gabapentin 600 mg)
Pill with imprint T 1 is White, Elliptical/Oval and has been identified as Gabapentin 600 mg. It is supplied by Camber Pharmaceuticals, Inc.
Gabapentin is used in the treatment of postherpetic neuralgia; epilepsy and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy.
Gabapentin 600 mg is not a controlled substance under the Controlled Substances Act (CSA).
Gabapentin
- Imprint
- T 1
- Strength
- 600 mg
- Color
- White
- Shape
- Elliptical/Oval
- Availability
- Prescription only
- Drug Class
- Gamma-aminobutyric acid analogs
- Pregnancy Category
- C – Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Camber Pharmaceuticals, Inc.
- Manufacturer
- Ascent Pharmaceuticals, Inc.
- National Drug Code (NDC)
- 31722-0166
- Inactive Ingredients
- mannitol, hydroxypropyl cellulose, crospovidone, talc, silicon dioxide, glyceryl dibehenate, magnesium stearate
-
T 3 (Gabapentin 800 mg)
Pill with imprint T 3 is White, Elliptical/Oval and has been identified as Gabapentin 800 mg. It is supplied by Camber Pharmaceuticals, Inc.
Gabapentin is used in the treatment of postherpetic neuralgia; epilepsy and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 800 mg is not a controlled substance under the Controlled Substances Act (CSA).
Gabapentin
- Imprint
- T 3
- Strength
- 800 mg
- Color
- White
- Shape
- Elliptical/Oval
- Availability
- Prescription only
- Drug Class
- Gamma-aminobutyric acid analogs
- Pregnancy Category
- C – Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Camber Pharmaceuticals, Inc.
- Manufacturer
- Ascent Pharmaceuticals, Inc.
- National Drug Code (NDC)
- 31722-0167
- Inactive Ingredients
- mannitol, hydroxypropyl cellulose, crospovidone, talc, silicon dioxide, glyceryl dibehenate, magnesium stearate